Description
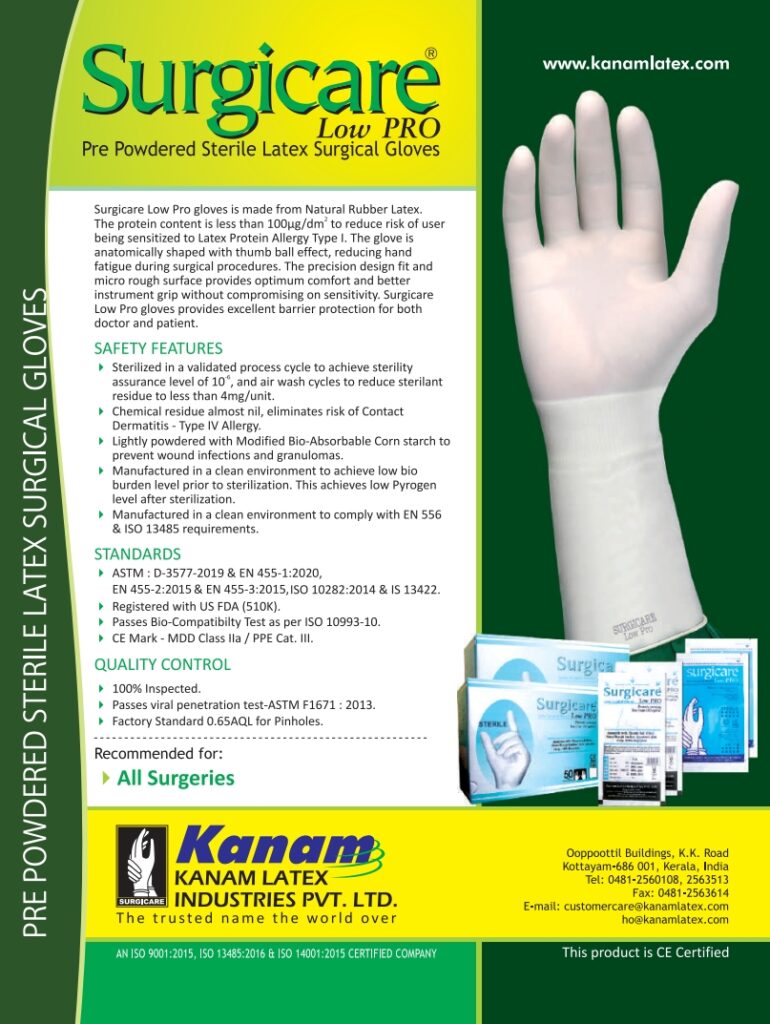
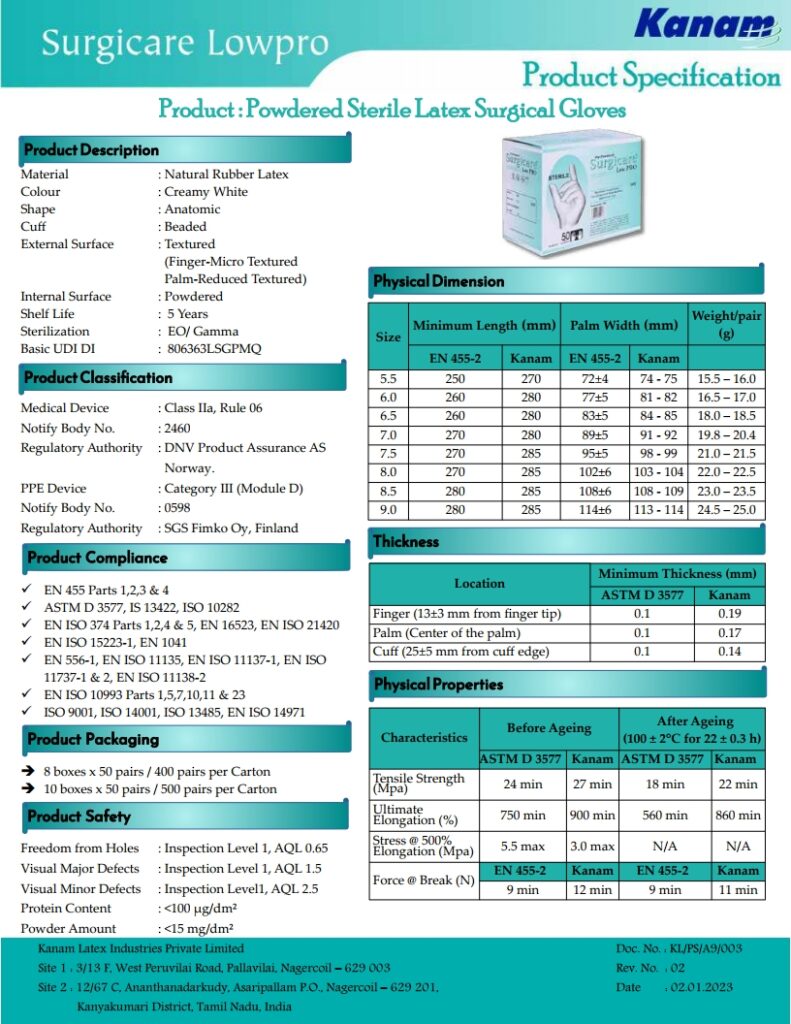
Surgicare®Low Pro gloves is made from Natural Rubber Latex. The protein content is less than 100μg/dm2 to reduce risk of user being sensitized to Latex Protein Allergy Type I. The glove is anatomically shaped with thumb ball effect, reducing hand fatigue during surgical procedures. The precision design fit and micro rough surface provides optimum comfort and better instrument grip without compromising on sensitivity. Surgicare Low Pro gloves provides excellent barrier protection for both doctor and patient.
Safety Features
- Sterilized in a validated process cycle to achieve sterility assurance level of 10-6, and air wash cycles to reduce sterilant residue to less than 4mg/unit.
- Chemical residue almost nil, eliminates risk of Contact Dermatitis - Type IV Allergy.
- Lightly powdered with Modified Bio-Absorbable Corn starch to prevent wound infections and granulomas.
- Manufactured in a clean environment to achieve low bio burden level prior to sterilization. This achieves low Pyrogen level after sterilization.
- Manufactured in a clean environment to comply with EN 556 & ISO 13485 requirements.
Standards
- ASTM : D-3577-2019 & EN 455-1:2020, EN 455-2:2015 & EN 455-3:2015, ISO 10282:2014 & IS 13422.
- Registered with US FDA (510K).
- Passes Bio-Compatibilty Test as per ISO 10993-10.
- CE Mark - MDD Class Ila / PPE Cat. III.
Quality
- 100% Inspected.
- Passes viral penetration test-ASTM F1671: 2013.
- Factory Standard 0.65AQL for Pinholes.
Recommended for all surgical procedure
All Surgeries